Background: Heavy menstrual bleeding (HMB) is the dominant symptom affecting ~80-90% of women with VWD and is a common cause of outpatient visits and hospitalizations. Long-term prophylaxis with a von Willebrand factor (VWF) concentrate is recommended in von Willebrand disease (VWD) patients with a history of severe, frequent bleeds, and for women with VWD who experience HMB. A recent prospective study found that recombinant VWF was not superior to tranexamic acid at reducing HMB in women with VWD. The WIL-31 study demonstrated the efficacy of prophylaxis with a plasma-derived VWF/factor VIII (pdVWF/FVIII) concentrate containing VWF and FVIII in a 1:1 activity ratio (wilate ®) in adults and children with VWD of all types. The primary endpoint was met, showing a decrease of 84% in the mean total annualized bleeding rate (ABR) compared with prior on-demand treatment. The impact of pdVWF/FVIII prophylaxis on HMB was evaluated as a secondary endpoint of the WIL-31 study.
Aims: To investigate the efficacy of regular prophylaxis with a pdVWF/FVIII concentrate in reducing the incidence of HMB in females with VWD who had previously been treated on demand.
Methods: WIL-31 (NCT04052698) was a prospective, non-controlled, international, multicenter phase 3 trial that enrolled male/female patients, aged ≥6 years old with VWD type 1 (VWF:RCo <30 IU/dL), type 2 (except 2N) or type 3. Prior to entering the WIL-31 study, all patients had received on-demand treatment with a VWF-containing product during a 6-month, prospective, observational, run-in study (WIL-29). Patients in WIL-31 received regular pdVWF/FVIII prophylaxis 2-3 times per week at a dose of 20-40 IU/kg for 12 months. Exploratory analyses included comparison of heavy menstrual ABR (HMABR). HMB was defined as any menstrual bleed that impeded the ability to perform daily activities (such as work, housework, exercise, or social activities) during menstrual periods. Criteria for HMB included changing pads more frequently than hourly, menstrual bleeding lasting 7 or more days, or the presence of clots >1 cm combined with a history of flooding or a Pictorial Blood Assessment Chart (PBAC) score ≥185. PBAC scores were reviewed from patient diaries at each study visit.
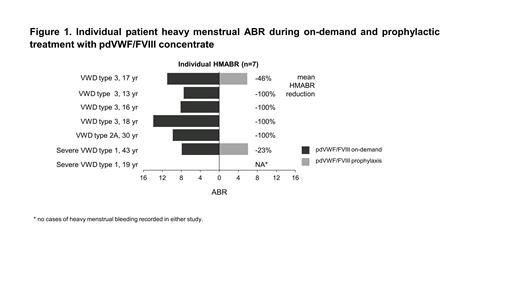
Results: Of the 33 patients included in the overall population, 14 (42%) were female and half (n=7; 13-43 years old) were of childbearing age. In these females, all VWD types were represented: type 1 (n=2), type 2 (n=1); and type 3 (n=4). Six (86%) females were on hormonal birth control during WIL-31. Prophylaxis with pdVWF/FVIII reduced the mean HMABR by 64% compared with on-demand treatment (3.0 vs 8.3 in WIL-31 and WIL-29, respectively). During on-demand treatment, 86% (6/7) of females experienced ≥1 HMB episode, which was reduced to 29% (2/7) during prophylaxis with four patients experiencing no HMB (Figure 1). During on-demand treatment, 6 females experienced 32 HMB episodes; of these, 7 (22%) required further treatment with pdVWF/FVIII and 2 (6%) required concomitant medication. Under prophylaxis, two patients (aged ≥17 years), experienced 12 HMBEs; of these, only one (8%) impeded daily activity and none required additional pdVWF/FVIII treatment, hospitalization, or concomitant medication. Mean (SD) intra-individual median PBAC scores (N=4) decreased by 43% from 227 (91.7) during on-demand treatment to 131 (59.9) with prophylaxis, a 96-point reduction.
Conclusion: Prophylaxis with pdVWF/FVIII was efficacious in reducing HMB compared with on-demand treatment in 7 female patients with VWD. None of the cases of HMB during prophylaxis was severe enough to require additional treatment. Among women with VWD, HMB remains a major burden and further research is required.
OffLabel Disclosure:
Kiss:Novo Nordisk Hungaria: Honoraria; Takeda: Honoraria; H-W-H: Honoraria; Sobi: Honoraria; Roche Hungary: Honoraria; CSL Behring: Honoraria. Boda:Novo Nordisk: Research Funding; Takeda: Research Funding. Khayat:LFB: Honoraria; CSL Behring: Honoraria; Octapharma: Honoraria. Dubey:Octapharma: Other: Clinical study investigator . Sidonio:Bayer: Honoraria; Takeda: Honoraria, Research Funding; Guardian Therapeutics: Honoraria; Novo Nordisk: Honoraria; UniQure: Honoraria; Biomarin: Honoraria; Spark: Honoraria; Pfizer: Honoraria; Octapharma: Honoraria, Research Funding; Genentech: Honoraria, Research Funding.
This study is investigating the use of a VWF / FVIII concentrate for the prevention of bleeds in patients with von Willebrand disease of any type

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal